| 3/19/2024 |
Proposed Rule |
Drug Products or Categories of Drug Products That Present Demonstrable Difficulties for Compounding Under Sections 503A or 503B of the Federal Food, Drug, and Cosmetic Act |
N/A |
| 3/11/2024 |
Guidance |
Temporary Policies for Compounding Certain Parenteral Drug Products; List for parenteral drug products compounded by pharmacy compounders |
Hurricane Helene: Baxter’s manufacturing recovery in North Carolina |
| 12/7/2023 |
Draft Guidance |
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
N/A |
| 12/7/2023 |
Draft Guidance |
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503A of the Federal Food, Drug, and Cosmetic Act |
N/A |
| 8/21/2023 |
Federal Register Notice |
List of Bulk Drug Substances for Which There is a Clinical Need Under Section 503B the Federal Food, Drug, and Cosmetic Act |
N/A |
| 6/27/2023 |
Guidance |
Prohibition on Wholesaling Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
N/A |
| 4/6/2023 |
Federal Register Notice |
List of Bulk Drug Substances for Which There Is a Clinical Need Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
Compounding Safety Information: Quinacrine Hydrochloride |
| 2/9/2023 |
Guidance |
Compounding Certain Ibuprofen Oral Suspension Products Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
FDA issues guidance to help increase supply of ibuprofen oral suspension products in hospitals and health systems
Questions and Answers on Compounded Oral Suspension Medications for Pain and Fever
|
| 11/23/2022 |
Federal Register Notice |
List of Bulk Drug Substances for Which There is a Clinical Need Under Section 503B the Federal Food, Drug, and Cosmetic Act |
N/A |
| 11/23/2022 |
Immediately in Effect Guidance |
Compounding Certain Beta-Lactam Products in Shortage Under Section 503A of the Federal Food, Drug, and Cosmetic Act |
N/A |
| 10/21/2022 |
Federal Register Notice |
Extension of the Period Before the Food and Drug Administration Intends To Begin Enforcing the Statutory 5 Percent Limit on Out-of-State Distribution of Compounded Human Drug Products |
N/A |
| 1/21/2022 |
Federal Register Notice |
List of Bulk Drug Substances for Which There Is a Clinical Need Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
FDA Roundup: January 28, 2022 |
| 10/6/2021 |
Revised Draft Guidance |
Hospital and Health System Compounding Under Section 503A of the Federal Food, Drug, and Cosmetic Act |
FDA Revises Hospital and Health System Compounding Guidance to Help Preserve Patient Access to Compounded Drugs |
| 8/9/2021 |
Federal Register Notice |
Extension of the Period Before the Food and Drug Administration Intends To Begin Enforcing the Statutory 5 Percent Limit on Out of State Distribution of Compounded Human Drug Products |
N/A |
| 3/23/2021 |
Federal Register Notice |
List of Bulk Drug Substances for Which There Is a Clinical Need Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
FDA Provides Preliminary Assessment on Five Bulk Drug Substances for Use by Outsourcing Facilities |
| 11/6/2020 |
Final Guidance |
Insanitary Conditions at Compounding Facilities
Notice of Availability
|
FDA Takes Efforts to Protect Patients from Potentially Harmful Compounded Drugs Through Finalizing Insanitary Conditions Guidance
CDER’s Efforts to Help Improve the Quality of Drug Compounding Include an Ongoing Focus on Insanitary Conditions
|
| 10/26/2020 |
Final MOU |
Final Standard Memorandum of Understanding Addressing Certain Distributions of Compounded Drug Products Between the [insert STATE BOARD OF PHARMACY or OTHER APPROPRIATE STATE AGENCY] and the U.S. Food and Drug Administration
Notice of availability
|
FDA in Brief: FDA Announces Significant Milestone in Compounding Program to Protect Public Health Through Collaboration with States |
| 10/26/2020 |
Final MOU |
Final Standard Memorandum of Understanding Addressing Certain Distributions of Compounded Drug Products Between the [insert STATE BOARD OF PHARMACY or OTHER APPROPRIATE STATE AGENCY] and the U.S. Food and Drug Administration |
FDA in Brief: FDA Announces Significant Milestone in Compounding Program to Protect Public Health Through Collaboration with States |
| 8/19/2020 |
Federal Register Notice |
Registration of Human Drug Compounding Outsourcing Facilities Under Section 503B of the Federal Food, Drug, and Cosmetic Act and Associated Fees |
N/A |
| 7/30/2020 |
Federal Register Notice |
List of Bulk Drug Substances for Which There is a Clinical Need Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
FDA in brief: FDA Proposes Four Bulk Drug Substances for Inclusion, 19 Not to Be Included on 503B Bulks List for Use by Outsourcing Facilities |
| 5/13/2020 |
Federal Register Notice |
Agency Information Collection Activities; Submission for Office of Management and Budget Review; Comment Request; Human Drug Compounding Under Sections 503A and 503B of the Federal Food, Drug, and Cosmetic Act |
FDA Announces Latest Step Toward Finalizing Memorandum of Understanding with States Addressing Compounded Drug Distribution, While Preserving Access |
| 4/8/2020 |
Final Guidance |
Postmarketing Adverse Event Reporting for Medical Products and Dietary Supplements During a Pandemic |
N/A |
| 1/22/2020 |
Revised Draft Guidance |
Current Good Manufacturing Practice—Guidance for Human Drug Compounding Outsourcing Facilities |
N/A |
| 9/4/2019 |
Proposed Rule |
Amendments to the List of Bulk Drug Substances that can be used to Compound Drug Products in Accordance with Section 503A of the Federal Food, Drug, and Cosmetic Act |
N/A |
| 8/30/2019 |
Federal Register |
Notice List of Bulk Drug Substances for Which There is a Clinical Need Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
FDA seeks comment on its preliminary finding that there is no clinical need for outsourcing facilities to compound drugs using nine bulk drug substances |
| 5/23/2019 |
Guidance |
Guidance Section 503A Bulks List Final Rule Questions and Answers Guidance for Industry (Small Entity Compliance Guide)
Notice of Availability
|
N/A |
| 7/5/2019 |
Final Guidance |
Compliance Policy for Certain Compounding of Oral Oxitriptan (5-HTP) Drug Products for Patients With Tetrahydrobiopterin (BH4) Deficiency Immediately in Effect Guidance for Industry
Notice of Availability
|
FDA issues guidance on compounding oral oxitriptan (5-HTP) for patients with tetrahydrobiopterin (BH4) deficiency |
| 3/1/2019 |
Federal Register Notice |
List of Bulk Drug Substances for which there is a Clinical Need Under Section 503Bof the Federal Food, Drug, and Cosmetic Act |
FDA finalizes guidance on evaluating the Clinical Need for outsourcing facilities to compound drugs with bulk drug substances; provides final decision on two substances |
| 3/1/2019 |
Final Guidance |
Evaluation of Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act |
N/A |
| 2/15/2019 |
Final Rule |
List of Bulk Drug Substances that can be used to Compound Drug Products in Accordance with Section 503Athe Federal Food, Drug, and Cosmetic Act |
FDA continues to advance oversight of drug compounding by finalizing a rule providing information on several bulk drug substances compounders not registered as outsourcing facilities can and cannot use |
| 12/10/2018 |
Federal Register Notice of Public Meeting |
The Food and Drug Administration’s Proposed Current Good Manufacturing Practice Policies for Outsourcing Facilities: Considerations Regarding Access to Office Stock |
Statement from FDA Commissioner Scott Gottlieb, M.D. and Deputy Commissioner Anna Abram on new efforts to assure the quality of compounded drugs |
| 12/10/2018 |
Final Rule |
List of Drug Products That Have Been Withdrawn or Removed From the Market for Reasons of Safety or Effectiveness |
N/A |
| 9/25/2018 |
Final Guidance |
Compounding and Repackaging of Radiopharmaceuticals by State-Licensed Nuclear Pharmacies and Federal Facilities |
N/A |
| 9/25/2018 |
Final Guidance |
Compounding and Repackaging of Radiopharmaceuticals By Outsourcing Facilities |
N/A |
| 8/27/2018 |
Federal Register Notice |
List of Bulk Drug Substances That Can Be Used to Compound Drug Products in Accordance With Section 503B of the Federal Food, Drug, and Cosmetic Act |
FDA seeks comment on its preliminary finding that there is no clinical need for outsourcing facilities to compound from three bulk drug substances that are ingredients in FDA-approved drugs |
| 5/10/2018 |
Final Guidance |
Facility Definition Under Section 503B of the Federal Food, Drug, and Cosmetic Act; Guidance for Industry
Notice of Availability
|
FDA in Brief: FDA issues new policy on what constitutes an outsourcing facility, a key step in implementing the agency’s comprehensive framework for compounding |
| 1/18/2018 |
Final Guidance |
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act
Notice of Availability
|
FDA in Brief: 2018 Compounding Policy Priorities Plan |
| 1/18/2018 |
Final Guidance |
Compounded Drug Products That Are Essentially Copies of Approved Drug Products Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Notice of Availability
|
FDA in Brief: 2018 Compounding Policy Priorities Plan |
| 1/18/2018 |
Final Guidance |
Mixing, Diluting, or Repackaging Biological Products Outside the Scope of an Approved Biologics License Application
Notice of Availability
|
FDA in Brief: 2018 Compounding Policy Priorities Plan |
| 7/28/2017 |
FR Notice |
Drug Products That Present Demonstrable Difficulties for Compounding Under the Federal Food, Drug, and Cosmetic Act; Establishment of a Public Docket |
N/A |
| 1/13/2017 |
Final Guidance |
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry (PDF – 126KB)
Notice of Availability
|
FDA issues final guidances on interim policy for certain bulk drug substances used in compounding |
| 1/13/2017 |
Final Guidance |
Interim Policy on Compounding Using Bulk Drug Substances Under Section 503B of the Federal Food, Drug, and Cosmetic Act Guidance for Industry (PDF – 105KB)
Notice of Availability
|
FDA issues final guidances on interim policy for certain bulk drug substances used in compounding |
| 1/12/2017 |
Final Guidance |
Repackaging of Certain Human Drug Products by State-Licensed Pharmacies and Outsourcing Facilities
Notice of Availability
|
FDA Issues Final Guidance on Repackaging and Revised Draft Guidance on Mixing, Diluting, and Repackaging Biological Products |
| 12/30/2016 |
Final Guidance |
Electronic Drug Product Reporting for Human Drug Compounding Outsourcing Facilities Under Section 503B of the Federal Food, Drug, and Cosmetic Act (PDF – 191KB)
Notice of Availability
|
N/A |
| 12/28/2016 |
Final Guidance |
Prescription Requirement Under Section 503A of the Federal Food, Drug, and Cosmetic Act
Notice of Availability
|
FDA issues guidance on prescription requirement under section 503A |
| 10/17/2016 |
Proposed Rule |
Amendments to the Regulation Regarding the List of Drug Products That Have Been Withdrawn or Removed From the Market for Reasons of Safety or Effectiveness
Notice of Availability
|
Proposed rule to amend the list of drug products that may not be compounded because they have been withdrawn or removed from the market for safety and effectiveness reasons |
| 10/6/2016 |
Final Rule |
Additions and Modifications to the List of Drug Products That Have Been Withdrawn or Removed From the Market for Reasons of Safety or Effectiveness
Notice of Availability
|
Final rule amending list of drug products that may not be compounded because they have been withdrawn or removed from the market for safety or effectiveness reasons |
| 6/9/2016 |
Final Guidance |
Pharmacy Compounding of Human Drug Products Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance (PDF – 86KB) |
N/A |
| 4/15/2016 |
Draft Guidance |
Hospital and Health System Compounding Under the Federal Food, Drug, and Cosmetic Act
Notice of Availability
|
FDA issues three new draft guidances related to compounding of human drugs |
| 10/26/2015 |
Request for Nominations |
Bulk Drug Substances That Can Be Used To Compound Drug Products in Accordance With Section 503B of the Federal Food, Drug, and Cosmetic Act; Establishment of a Public Docket |
N/A |
| 10/26/2015 |
Request for Nominations |
Bulk Drug Substances That Can Be Used To Compound Drug Products in Accordance With Section 503A of the Federal Food, Drug, and Cosmetic Act; Establishment of a Public Docket |
N/A |
| 10/8/2015 |
Final Guidance |
Adverse Event Reporting for Outsourcing Facilities Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Notice of Availability
|
N/A |
| 8/12/2015 |
Final Guidance |
Guidance For Entities Considering Whether to Register As Outsourcing Facilities Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Notice of Availability
|
N/A |
| 3/6/2015 |
Public Docket |
Compounding of Human Drug Products Under Sections 503A and 503B of the Federal Food, Drug, and Cosmetic Act; Establishment of a Public Docket |
FDA Establishes Public Docket on Drug Compounding |
| 11/21/2014 |
Final Guidance |
Fees for Human Drug Compounding Outsourcing Facilities Under the FD&C Act
Notice of Availability
|
FDA issues additional guidance for outsourcing facilities that compound sterile human drugs |
Source
 Kawaii Animals Medical Patches 120 pcs Set
$4.99 – $7.99Price range: $4.99 through $7.99
Kawaii Animals Medical Patches 120 pcs Set
$4.99 – $7.99Price range: $4.99 through $7.99
 Active Noise Cancelling Headphone With Carrying Bag
$44.99 – $58.99Price range: $44.99 through $58.99
Active Noise Cancelling Headphone With Carrying Bag
$44.99 – $58.99Price range: $44.99 through $58.99
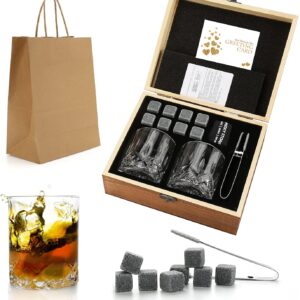 Whiskey Chilling Rocks in Wooden Box
$25.99 – $50.99Price range: $25.99 through $50.99
Whiskey Chilling Rocks in Wooden Box
$25.99 – $50.99Price range: $25.99 through $50.99
 Massage Seat Cushion
$6.99 – $24.99Price range: $6.99 through $24.99
Massage Seat Cushion
$6.99 – $24.99Price range: $6.99 through $24.99
 Facial Collagen Tablets for Fruit Mask Maker Machine
$8.99 – $13.99Price range: $8.99 through $13.99
Facial Collagen Tablets for Fruit Mask Maker Machine
$8.99 – $13.99Price range: $8.99 through $13.99