BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Feb 12, 2026 | News
Summary Company Announcement Date: February 11, 2026 FDA Publish Date: February 12, 2026 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description Listeria monocytogenes Company Name: Slade Gorton & Co., Inc. Brand Name: Product...by Pill Pals Customer Service | Feb 12, 2026 | News
March 12, 2026The meeting presentations will be heard, viewed, captioned, and recorded through an online teleconferencing platform.On March 12, 2026, the Committee will meet in open session to discuss and make recommendations on the strain composition of influenza...by Pill Pals Customer Service | Feb 12, 2026 | News
Prescription stimulants are classified as Schedule II drugs under the Controlled Substances Act and associated with serious risks, including misuse, addiction, overdose, and diversion. Stimulants are an area of great interest given that the number of prescriptions has...by Pill Pals Customer Service | Feb 12, 2026 | News
[2-11-2026] The Food and Drug Administration is advising consumers not to purchase or use Rhino Choco VIP Chocolate for Men, a product promoted and sold for sexual enhancement on various websites, including usaless.com, and possibly in some retail stores. FDA...by Pill Pals Customer Service | Feb 11, 2026 | News
[2-11-2026] The Food and Drug Administration is advising consumers not to purchase or use ilum Male Sexual Enhancement Chocolate, a product promoted and sold for sexual enhancement on various websites, including ebay.com, and possibly in some retail stores. FDA...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
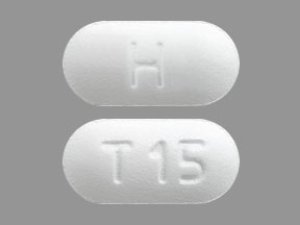 Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
 Sildenafil 25 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 25 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
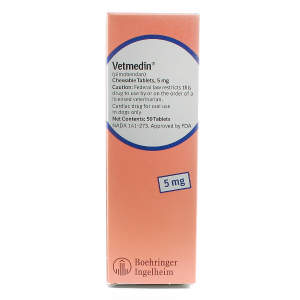 VETMEDIN CHEW 5 MG --- Pimobendan--- Boehringer Ingelheim Pharma
VETMEDIN CHEW 5 MG --- Pimobendan--- Boehringer Ingelheim Pharma
 Sildenafil 100 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 100 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
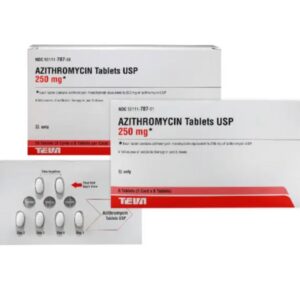 Azithromycin 250 mg Tablet Six (6) Pack --- TEVA Pharma --- Generic For Z Pack / Zithromax
Azithromycin 250 mg Tablet Six (6) Pack --- TEVA Pharma --- Generic For Z Pack / Zithromax