BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Feb 18, 2026 | News
Emerging Technology Program Graduates its First TechnologyThe Emerging Technology Program (ETP) is proud to announce it graduated its first technology, Continuous Direct Compression (CDC) on October 21, 2021. Graduation is the process through which ETP transfers...by Pill Pals Customer Service | Feb 18, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Christian Medical College, Vellore, India (C.S. Devamani, W. Rose, K.P.P....by Pill Pals Customer Service | Feb 18, 2026 | News
Summary Company Announcement Date: February 13, 2026 FDA Publish Date: February 17, 2026 Product Type: Dietary Supplements Reason for Announcement: Recall Reason Description Product contains 7-Hydroxymitragynine (7-OH) in an amount more than the declared value of 7.5...by Pill Pals Customer Service | Feb 17, 2026 | News
Delivery Method: VIA EMAIL WITH READ RECEIPT Reference #: 320-26-43 Product: Drugs Recipient: Recipient Name Mr. Valentine P. Fittler Recipient Title CEO & Managing Director Cosmetic Manufacturers Pty Ltd. PO Box 5767Queensland Mail CentreBundall QLD...by Pill Pals Customer Service | Feb 17, 2026 | News
Delivery Method: VIA UNITED PARCEL SERVICE AND VIA E-MAIL Reference #: 26-HFD-45-02-02 Product: Drugs Recipient: Recipient Name Bertrand P. Cole, D.O. Bertrand P. Cole, D.O./Activmed Practices and Research, LLC 110 Corporate Drive, Suite 2Portsmouth, NH 03801United...by Pill Pals Customer Service | Feb 17, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Mediterranean and Black Sea Programme in Intervention Epidemiology...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!


 Sildenafil 50 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 50 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
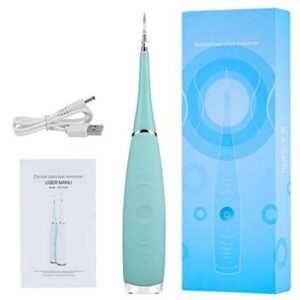 Portable Electric Dental Scaler Tool
$6.99 – $10.99Price range: $6.99 through $10.99
Portable Electric Dental Scaler Tool
$6.99 – $10.99Price range: $6.99 through $10.99
 Acetaminophen 500 MG Caplets ---1,000 Count --- Generic For Tylenol --- Major Pharma
Acetaminophen 500 MG Caplets ---1,000 Count --- Generic For Tylenol --- Major Pharma
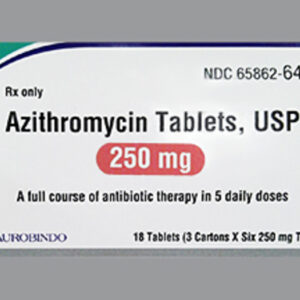 Azithromycin 250 mg Tablet Six (6) Pack --- Aurobindo Pharma --- Generic For Z Pack / Zithromax
Azithromycin 250 mg Tablet Six (6) Pack --- Aurobindo Pharma --- Generic For Z Pack / Zithromax
 Oxymetazoline Nasal Decongestant Spray 12 hr (3 PACK) --- Generic For Afrin --- RELIABLE 1 (Reliable One)
Oxymetazoline Nasal Decongestant Spray 12 hr (3 PACK) --- Generic For Afrin --- RELIABLE 1 (Reliable One)