BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Feb 9, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Centers for Disease Control and Prevention, Atlanta, Georgia, USA Juan...by Pill Pals Customer Service | Feb 9, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: University of Leicester Medical School, Leicester, United Kingdom Mumps...by Pill Pals Customer Service | Feb 7, 2026 | News
Summary Company Announcement Date: February 06, 2026 FDA Publish Date: February 06, 2026 Product Type: Medical Devices Reason for Announcement: Recall Reason Description As currently written, the Owner’s Booklets/System Instructions for Use fails to emphasize that...by Pill Pals Customer Service | Feb 6, 2026 | News
510(k) Number: BK251168Applicant: Arteriocyte Medical Systems, Inc. DBA Isto BiologicsDevice Name: Precise Cell Concentration SystemDecision Date: 02/03/2026Supporting Documents Content current as of: 02/05/2026...by Pill Pals Customer Service | Feb 6, 2026 | News
The U.S. Food and Drug Administration (FDA) is providing this communication to increase awareness of recent updates to the product labeling of capecitabine (Xeloda) and fluorouracil (5-FU) related to risks associated with dihydropyrimidine dehydrogenase (DPD)...by Pill Pals Customer Service | Feb 6, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Emory University School of Medicine, Atlanta, Georgia, USA (S. Bellman,...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
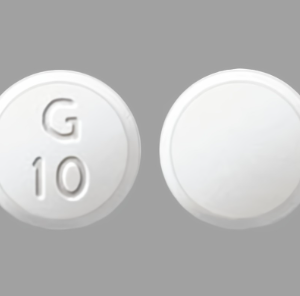
 Sildenafil 100 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 100 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
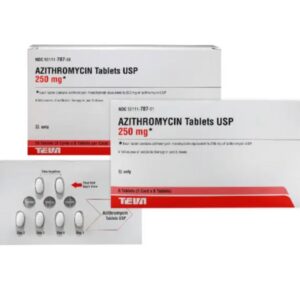 Azithromycin 250 mg Tablet Six (6) Pack --- TEVA Pharma --- Generic For Z Pack / Zithromax
Azithromycin 250 mg Tablet Six (6) Pack --- TEVA Pharma --- Generic For Z Pack / Zithromax
 12 Pack - My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill / Pack Of 12
12 Pack - My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill / Pack Of 12
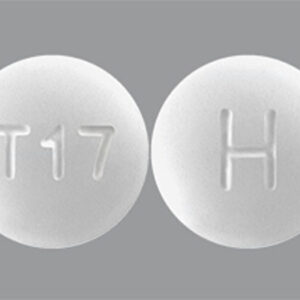 Tadalafil 5 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Tadalafil 5 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
 Sildenafil 100 mg Tablets --- Generic for Viagra --- Ajanta Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 100 mg Tablets --- Generic for Viagra --- Ajanta Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%