BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Oct 7, 2025 | News
Summary Company Announcement Date: October 07, 2025 FDA Publish Date: October 07, 2025 Product Type: Medical Devices Reason for Announcement: Recall Reason Description Potential for defective LCD displays that can affect product performance Company Name: Trividia...by Pill Pals Customer Service | Oct 7, 2025 | News
Summary Company Announcement Date: October 06, 2025 FDA Publish Date: October 07, 2025 Product Type: Food & Beverages Foodborne Illness Reason for Announcement: Recall Reason Description Potential to be contaminated with Listeria monocytogenes Company Name: Sno...by Pill Pals Customer Service | Oct 7, 2025 | News
ActionToday, the U.S. Food and Drug Administration (FDA) approved Jascayd (nerandomilast) tablets to treat idiopathic pulmonary fibrosis (IPF), a rare, serious, and progressive disease with no cure and limited treatments. This is the first new therapy approved in more...by Pill Pals Customer Service | Oct 7, 2025 | News
Product: Drugs Over-the-Counter Drugs Recipient: Recipient Name Rahul Patel Recipient Title President Centura Pharmaceuticals Inc 24718 State Road 54CLutz, FL 33559United States centurapharma@gmail.com Issuing Office: Center for Drug Evaluation and Research (CDER)...by Pill Pals Customer Service | Oct 7, 2025 | News
Delivery Method: VIA UPS Reference #: 320-25-115 Product: Drugs Over-the-Counter Drugs Recipient: Recipient Name Mr. Prakash C. Purohit Recipient Title President/Owner Naturich Cosmetique Labs 2505 Merritt DriveGarland, TX 75041-6158United States Issuing Office:...by Pill Pals Customer Service | Oct 7, 2025 | News
Delivery Method: VIA ELECTRONIC MAIL READ/DELIVERY RECEIPT REQUESTED Reference #: 320-25-116 Product: Drugs Over-the-Counter Drugs Recipient: Recipient Name Mr. Alex D. Daliva Recipient Title President Creative Essences, Inc. 15320 Cornet StreetSanta Fe Springs, CA...
 Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 5 --- Perrigo Pharma
Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 5 --- Perrigo Pharma
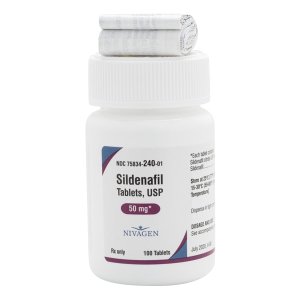 Sildenafil 50 mg Tablets --- Generic for Viagra --- Nivagen Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 50 mg Tablets --- Generic for Viagra --- Nivagen Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
 Sildenafil 25 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 25 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
 Tadalafil 10 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00
Tadalafil 10 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00
 Lidocaine Viscous Solution Two Percent (2%) 100 ml --- Akorn Pharma
Lidocaine Viscous Solution Two Percent (2%) 100 ml --- Akorn Pharma