BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Dec 13, 2025 | News
The Emerging Technology Program serves as an entry point for new technologies that will potentially be included in a regulatory filing for a specific drug product. As industry develops new technologies, they can reach out to ETP and request input and feedback on an...by Pill Pals Customer Service | Dec 13, 2025 | News
Drug master files (DMFs) are submissions to FDA used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of human drug products. DMFs:Allow parties to reference material...by Pill Pals Customer Service | Dec 13, 2025 | News
What are Quality Metrics? For pharmaceutical manufacturing, quality metrics are an objective way to measure, evaluate, and monitor the product and process lifecycle. Quality metrics data may lead to higher levels of safety, efficacy, delivery, and performance. ...by Pill Pals Customer Service | Dec 13, 2025 | News
A botanical drug product is intended for use in the diagnosis, cure, mitigation, treatment or prevention of disease in humans. A botanical drug product consists of vegetable materials, which may include plant materials, algae, macroscopic fungi, or combinations...by Pill Pals Customer Service | Dec 13, 2025 | News
ResourcesInspections Classifications DatabaseThis database provides final inspection classifications for inspections related to currently marketed FDA-regulated products, including inspections of facilities that manufacture, process, pack, or hold an FDA-regulated...by Pill Pals Customer Service | Dec 13, 2025 | News
The Center for Drug Evaluation and Research’s (CDER) Advanced Manufacturing Research Facility (AMRF) is a new laboratory for evaluating innovative drug manufacturing technologies and ensuring drugs produced with these technologies are safe, effective, and high...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
 Tadalafil 10 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
Tadalafil 10 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
 Automatic Sonic Electric U-Shaped Toothbrush
$11.99 – $24.99Price range: $11.99 through $24.99
Automatic Sonic Electric U-Shaped Toothbrush
$11.99 – $24.99Price range: $11.99 through $24.99
 Oxymetazoline Nasal Decongestant Spray 12 hr (3 PACK) --- Generic For Afrin --- Major Pharma
Oxymetazoline Nasal Decongestant Spray 12 hr (3 PACK) --- Generic For Afrin --- Major Pharma
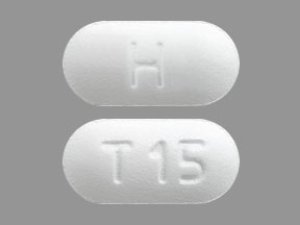 Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
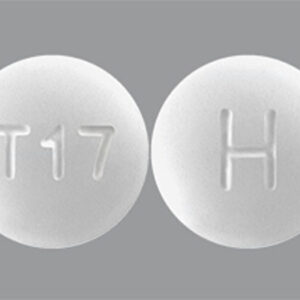 Tadalafil 5 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Tadalafil 5 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%