BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Dec 23, 2025 | News
This recall involves removing certain devices from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it.Affected Product The FDA is aware that B. Braun Medical...by Pill Pals Customer Service | Dec 23, 2025 | News
This recall involves removing certain devices from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it.Affected Product The FDA is aware that Medtronic has...by Pill Pals Customer Service | Dec 23, 2025 | News
Summary Company Announcement Date: December 22, 2025 FDA Publish Date: December 22, 2025 Product Type: Food & Beverages Allergens Reason for Announcement: Recall Reason Description Undeclared milk, wheat, and soy allergens Company Name: Troemner Farm Brand Name:...by Pill Pals Customer Service | Dec 23, 2025 | News
Should you find a link that does not work within any Guidance document, Rule or other document posted on the FDA Web site, please try searching for the document using the document title. If you need further assistance, please go to Contact FDA. Content current as of:...by Pill Pals Customer Service | Dec 23, 2025 | News
This recall involves updating instructions for using certain devices, and does not involve removing them from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use...by Pill Pals Customer Service | Dec 22, 2025 | News
Coagulation Factor Xa (Recombinant), Inactivated-ZHZO | FDA Source
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
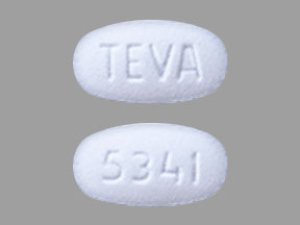 Sildenafil 25 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 25 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
 Acetaminophen 500 MG Caplets ---1,000 Count --- Generic For Tylenol --- Major Pharma
Acetaminophen 500 MG Caplets ---1,000 Count --- Generic For Tylenol --- Major Pharma
 Automatic Sonic Electric U-Shaped Toothbrush
$11.99 – $24.99Price range: $11.99 through $24.99
Automatic Sonic Electric U-Shaped Toothbrush
$11.99 – $24.99Price range: $11.99 through $24.99
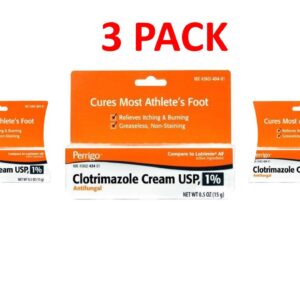 Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 3 --- Perrigo Pharma
Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 3 --- Perrigo Pharma
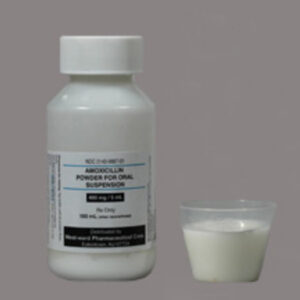 Amoxicillin 400 mg / 5 ml Suspension Liquid 100 ML --- Hikma Pharma
Amoxicillin 400 mg / 5 ml Suspension Liquid 100 ML --- Hikma Pharma