BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Feb 16, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Universidade de São Paulo, São Paulo, São Paulo, Brazil (A.N. Duarte-Neto,...by Pill Pals Customer Service | Feb 16, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Aurélie Truffot , Anne-Sophie Montcuquet, Gilda Grard, Laura Pezzi, Raphaelle Klitting,...by Pill Pals Customer Service | Feb 16, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Centre Hospitalier Universitaire, Besançon, France (C. Slekovec, A....by Pill Pals Customer Service | Feb 16, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: SMG-SNU Boramae Medical Center, Seoul, South Korea (S.H. Song, H.J. Kim);...by Pill Pals Customer Service | Feb 16, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Swine Viral Evolution and Vaccine Development Research Unit, Chulalongkorn...by Pill Pals Customer Service | Feb 16, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: University of Liverpool, Liverpool, UK (V.H. Sheridan, C.W. Duffy, L....
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!

 Sildenafil 25 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 25 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
 Docusate Sodium /Senna 8.6-50 mg Tablets 100 Count --- Generic for Peri-Colace --- Rugby Pharma
Docusate Sodium /Senna 8.6-50 mg Tablets 100 Count --- Generic for Peri-Colace --- Rugby Pharma
 3 Pack - My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill / Pack Of 3
3 Pack - My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill / Pack Of 3
 My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill
My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill
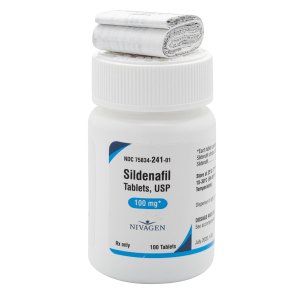 Sildenafil 100 mg Tablets --- Generic for Viagra --- Nivagen Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 100 mg Tablets --- Generic for Viagra --- Nivagen Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%