BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Feb 14, 2026 | News
On This Page Date: February 19, 2026 Time: 12:00 a.m. – 1:00 p.m. ET To AttendFor technical assistance please contact: Rakesh.Raghuwanshi@fda.hhs.govSpeaker Ronit Mazor, PhDPrincipal Investigator & CMC ReviewerGene Transfer and Immunogenicity BranchDivision...by Pill Pals Customer Service | Feb 14, 2026 | News
Delivery Method: Via Overnight Delivery Product: DrugsFood & Beverages Issuing Office: Center for Food Safety and Applied Nutrition (CFSAN) United States WARNING LETTER July 1, 2022 RE: 626291 Dear Mr. Mousstaid, This letter is to advise you that the U.S. Food...by Pill Pals Customer Service | Feb 13, 2026 | News
Product: DrugsFood & Beverages Recipient: Recipient Name Steven Hanley Nutrishus Brands, Inc. 3475 Oak Valley Rd NE, Unit 2150Atlanta, GA 30326United States Issuing Office: Center for Food Safety and Applied Nutrition (CFSAN) United States July 28, 2021 WARNING...by Pill Pals Customer Service | Feb 13, 2026 | News
Image The effects of menopause can make a woman’s daily life much harder, and therapies approved by the U.S. Food and Drug Administration can help. But too many women might not use these treatments to lessen their menopause symptoms because of the risks associated...by Pill Pals Customer Service | Feb 13, 2026 | News
[2-13-2026] The Food and Drug Administration is advising consumers not to purchase or use Boner Bears Chocolate, a product promoted and sold for sexual enhancement on various websites, including elyxr.com and lockoutsupplements.com, and possibly in some retail stores....by Pill Pals Customer Service | Feb 13, 2026 | News
[2-13-2026] The Food and Drug Administration is advising consumers not to purchase or use DTF Sexual Chocolate, a product promoted and sold for sexual enhancement on various websites, including shopsexology.com, and possibly in some retail stores. FDA laboratory...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
 Pataday - BRAND Eye Drops Allergy Itch Relief 0.2% by Alcon
Pataday - BRAND Eye Drops Allergy Itch Relief 0.2% by Alcon
 12 Pack - My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill / Pack Of 12
12 Pack - My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill / Pack Of 12
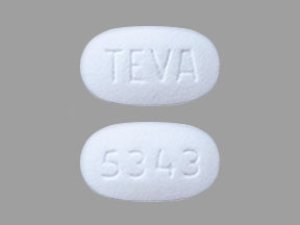 Sildenafil 100 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 100 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
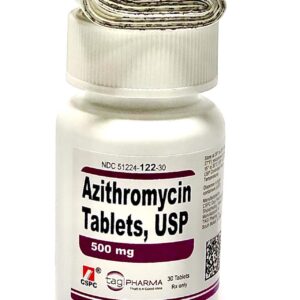 Azithromycin 500 mg Tablet Thirty (30) Pack TAGI Pharma --- Generic For Z Pack / Zithromax
Azithromycin 500 mg Tablet Thirty (30) Pack TAGI Pharma --- Generic For Z Pack / Zithromax
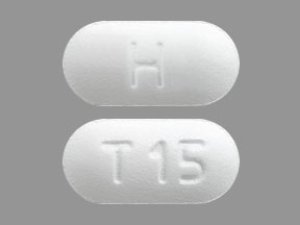 Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%