BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Oct 14, 2025 | News
Delivery Method: Via UPS and EMAIL Reference #: CBER 25-708912 Product: Biologics Recipient: Recipient Name William Reisacher, MD Recipient Title Senior Scientific Advisor and Co-Founder Allovate Therapeutics, LLC 20 W 125th St #1New York, NY 10027United States...by Pill Pals Customer Service | Oct 14, 2025 | News
Delivery Method: Certified Mail Reference #: 320-25-97 Product: Drugs Over-the-Counter Drugs Recipient: Recipient Name Dr. Sayed Ibrahim Recipient Title President and Chief Executive Officer Health and Natural Beauty USA Corp. 140 Ethel Rd West, Ste WPiscataway, NJ...by Pill Pals Customer Service | Oct 10, 2025 | News
Summary Company Announcement Date: October 10, 2025 FDA Publish Date: October 10, 2025 Product Type: Animal & VeterinaryFood & Beverages Pet Food Foodborne Illness Reason for Announcement: Recall Reason Description Potential Foodborne Illness –...by Pill Pals Customer Service | Oct 10, 2025 | News
AUDIENCE: Patient, Health Care Professional, Pharmacy, GastroenterologyISSUE: The FDA has received reports of immune effector cell-associated enterocolitis (IEC-EC) in patients who received treatment with CARVYKTI. Reports were received from clinical trials and...by Pill Pals Customer Service | Oct 10, 2025 | News
Who Is the Audience for This Website?FDA’s generic drug-specific labeling resources are primarily directed to industry staff who develop generic drug labeling. For more information about:Generic Drug LabelingGeneric drug labeling [labeling under an abbreviated new...by Pill Pals Customer Service | Oct 10, 2025 | News
In September 2022, the FDA User Fee Reauthorization Act of 2022 (FDAUFRA) was enacted, which included the third reauthorization of the Generic Drug User Fee Amendments (GDUFA). The current legislative authority for GDUFA III expires in September 2027. At that time,...by Pill Pals Customer Service | Oct 10, 2025 | News
Generic drug user fees make it possible for FDA and industry to continue to ensure that the American public has access to safe, effective and high-quality generic drugs. The implementation of the Generic Drug User Fee Amendments (GDUFA) encompasses a wide range of...by Pill Pals Customer Service | Oct 10, 2025 | News
This list includes applications for which we have approval documents available, and reflects the information as of the approval date. It is not updated with regard to applicant or application status changes. Information is arranged in alphabetical order by the name of...by Pill Pals Customer Service | Oct 10, 2025 | News
STN: BLA 125700Proper Name: nadofaragene firadenovec-vncgTradename: ADSTILADRINManufacturer: Ferring Pharmaceuticals A/SIndication: ADSTILADRIN is indicated for the treatment of adult patients with high-risk Bacillus Calmette-Guérin (BCG)-unresponsive non-Muscle...Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
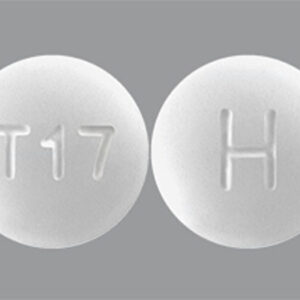
 Sildenafil 25 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 25 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
 Tadalafil 5 MG Tablets --- Generic for Cialis --- Ajanta Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Tadalafil 5 MG Tablets --- Generic for Cialis --- Ajanta Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
 Acetaminophen Liquid 160 MG / 5 ML - Generic for Tylenol 1 to 8 ounces
Acetaminophen Liquid 160 MG / 5 ML - Generic for Tylenol 1 to 8 ounces
 Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 5 --- Perrigo Pharma
Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 5 --- Perrigo Pharma
 Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF --- Perrigo Pharma
Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF --- Perrigo Pharma