BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Oct 10, 2025 | News
This recall involves correcting devices and does not involve removing them from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it without...by Pill Pals Customer Service | Oct 9, 2025 | News
Summary Company Announcement Date: October 09, 2025 FDA Publish Date: October 09, 2025 Product Type: Animal & VeterinaryFood & Beverages Foodborne Illness Reason for Announcement: Recall Reason Description Potential Foodborne Illness – Salmonella Company...by Pill Pals Customer Service | Oct 9, 2025 | News
Summary Company Announcement Date: October 08, 2025 FDA Publish Date: October 09, 2025 Product Type: Food & Beverages Foodborne Illness Reason for Announcement: Recall Reason Description Potential Foodborne Illness – Listeria monocytogenes Company Name: Sprouts...by Pill Pals Customer Service | Oct 9, 2025 | News
Generic Drugs Forum (GDF) 2024: Regulatory Considerations to Enhance Generic Drug Access – Day Two – Part OnePediatric Excipient Evaluation: Bioequivalence (BE) PerspectiveRecording and SlidesYang Lu, PhDSenior Staff FellowDB III | OB | OGD | CDERGDSA-BE: Modernizing...by Pill Pals Customer Service | Oct 8, 2025 | News
Summary Company Announcement Date: October 08, 2025 FDA Publish Date: October 08, 2025 Product Type: Food & Beverages Allergens Reason for Announcement: Recall Reason Description Undeclared shrimp allergen Company Name: TAI FOONG USA Brand Name: Product...by Pill Pals Customer Service | Oct 8, 2025 | News
STN:125720Proper Name: valoctocogene roxaparvovec-rvoxTradename: ROCTAVIANManufacturer: BioMarin Pharmaceutical Inc.Indication:Indicated for the treatment of adults with severe hemophilia A (congenital factor VIII deficiency with factor VIII activityby Pill Pals Customer Service | Oct 8, 2025 | News
On October 8, 2025, the Food and Drug Administration approved cemiplimab-rwlc (Libtayo, Regeneron Pharmaceuticals Inc.) for the adjuvant treatment of adults with cutaneous squamous cell carcinoma (CSCC) at high risk of recurrence after surgery and radiation.Full...by Pill Pals Customer Service | Oct 8, 2025 | News
Safety PharmacologyGeneral safety pharmacology testingSafety pharmacology endpoints may be incorporated into general toxicity studies in order to reduce animal use. This practice is routine for biologics. May include non-animal methods. May be based on a risk...by Pill Pals Customer Service | Oct 8, 2025 | News
Summary Company Announcement Date: October 06, 2025 FDA Publish Date: October 07, 2025 Product Type: Food & Beverages Spices, Flavors & Salts Contaminants Reason for Announcement: Recall Reason Description Potential Metal Contaminant – Lead Company...Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
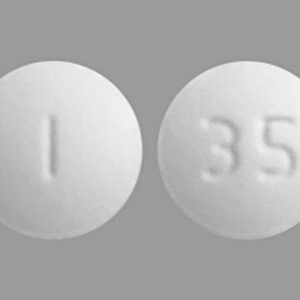
 Lidocaine Viscous Solution Two Percent (2%) 100 ml --- Hikma Pharma
Lidocaine Viscous Solution Two Percent (2%) 100 ml --- Hikma Pharma
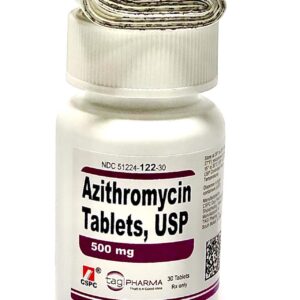 Azithromycin 500 mg Tablet Thirty (30) Pack TAGI Pharma --- Generic For Z Pack / Zithromax
Azithromycin 500 mg Tablet Thirty (30) Pack TAGI Pharma --- Generic For Z Pack / Zithromax
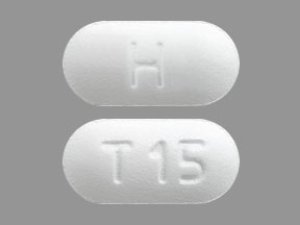 Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00
Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00
 Acetaminophen Liquid 160 MG / 5 ML - Generic for Tylenol 1 to 8 ounces
Acetaminophen Liquid 160 MG / 5 ML - Generic for Tylenol 1 to 8 ounces
 Portable Electric Dental Scaler Tool
$6.99 – $10.99Price range: $6.99 through $10.99
Portable Electric Dental Scaler Tool
$6.99 – $10.99Price range: $6.99 through $10.99