BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Sep 17, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Ulm University Hospital, Ulm, Germany (N. Gläser, N. Schwab, A. Osterloh,...by Pill Pals Customer Service | Sep 17, 2025 | News
Hand SanitizerNALIQUIDCardinal SpiritsAssay, ImpuritiesHand Sanitizer sampling and testingPassed Assay; Failed ImpuritiesHand SanitizerSeatex Hand Sanitizer Alcohol Antisepetic 80% Topical SolutionLIQUIDSeatex, LLCAssay, ImpuritiesHand Sanitizer sampling and...by Pill Pals Customer Service | Sep 17, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Daniel Evans , Hannah Friedlander, Khalid Bo-Subait, Jefferson Dennis, John Kaiyalethe,...by Pill Pals Customer Service | Sep 17, 2025 | News
FDA maintains a system of postmarket surveillance and risk assessment programs to identify and evaluate adverse drug reactions and medication errors that did not appear during the drug development process, and to learn more about known adverse drug reactions. As part...by Pill Pals Customer Service | Sep 17, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. John C. Cartee1, Thitima Cherdtrakulkiat12, Sandeep J. Joseph, Rossaphorn Kittiyaowamarn,...by Pill Pals Customer Service | Sep 17, 2025 | News
Directorio de Códigos Nacionales de Medicamentos (Spanish Version)FDA’s National Drug Code (NDC) Directory contains information about finished drug products, unfinished drugs and compounded drug products.Finished drug productsDrug establishments are required to...by Pill Pals Customer Service | Sep 17, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: University of Leipzig Medical Center, Leipzig, Germany (F. Baalmann, M....by Pill Pals Customer Service | Sep 17, 2025 | News
Host: Captain Mary KremznerPharmacist: Henry Yu, Pharm D.CAPT Kremzner: The “NDC Directory” locates unique National Drug Codes for marketed products. It’s a quick, easy, online resource.Hi, I’m Captain Mary Kremzner and this is Drug Info Rounds, brought to you by...by Pill Pals Customer Service | Sep 17, 2025 | News
Stop Distributing Unapproved Potassium Phosphates InjectionFDA requested a company stop distributing unapproved versions of potassium phosphates injection on August 1, 2024.The agency approved:Fresenius Kabi’s potassium phosphates injection (NDCs 65219-052, 65219-054...Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
 Oxymetazoline Nasal Decongestant Spray 12 hr (3 PACK) --- Generic For Afrin --- Major Pharma
Oxymetazoline Nasal Decongestant Spray 12 hr (3 PACK) --- Generic For Afrin --- Major Pharma
 6 Pack - My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill / Pack Of 6
6 Pack - My Way (Levonorgestrel) 1.5 mg Tablet - Generic For Plan B and Morning After Pill / Pack Of 6
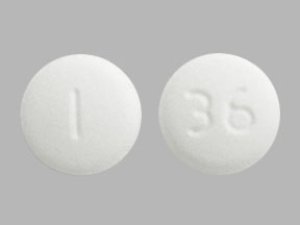 Sildenafil 50 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 50 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
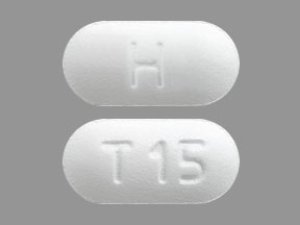 Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00
Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00
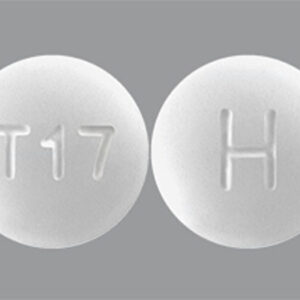 Tadalafil 5 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Tadalafil 5 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99