BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Sep 17, 2025 | News
[Posted 01/22/2025]AUDIENCE: Patient, Caregiver, Health Care Professional, Pharmacy, NeurologyISSUE: The FDA is warning about the risk of a rare but serious allergic reaction with the medicine glatiramer acetate (Copaxone, Glatopa), which is used to treat patients...by Pill Pals Customer Service | Sep 17, 2025 | News
Patients should stop taking glatiramer acetate and seek immediate medical attention by going to an emergency room or calling 911 if you experience symptoms of an anaphylactic reaction. Symptoms generally appear within one hour of injection and include wheezing or...by Pill Pals Customer Service | Sep 17, 2025 | News
Generic medications are just as safe and effective as their brand-name counterparts, and often cost less. As 9 in 10 prescriptions filled are for generics, people may have questions about how they are the same as the name brand medications and why there is a cost...by Pill Pals Customer Service | Sep 17, 2025 | News
Generic drugs are copies that one company makes of a brand-name drug that was developed by another company. Generally, generic drugs sell at lower prices, and it is in the public’s interest to get generic drugs to the market quickly. But, like any other...by Pill Pals Customer Service | Sep 17, 2025 | News
The FDA Office of Generic Drugs follows a rigorous review process to make sure that, compared to the brand-name (or innovator) medications, the proposed generic medications:Contain the same active/key ingredient;Have the same strength;Use the same dosage form (for...by Pill Pals Customer Service | Sep 17, 2025 | News
Recipient: Recipient Name Josh Disbrow Recipient Title Chief Executive Officer Aytu Biopharma 7900 E. Union Avenue, Suite 920Denver, CO 80237United States Issuing Office: Center for Drug Evaluation and Research (CDER) United States Secondary Issuing Offices WARNING...by Pill Pals Customer Service | Sep 17, 2025 | News
Delivery Method: VIA ELECTRONIC MAIL READ/DELIVERY RECEIPT REQUESTED Product: Drugs Issuing Office: Center for Drug Evaluation and Research (CDER) United States Secondary Issuing Offices WARNING LETTERSeptember 9, 2025FWD:This letter is to advise you that the United...by Pill Pals Customer Service | Sep 17, 2025 | News
Recipient: Recipient Name Maziar Mike Doustdar Recipient Title President and Chief Executive Officer Novo Nordisk Inc. 800 Scudders Mill RoadPlainsboro, NJ 08536United States Issuing Office: Center for Drug Evaluation and Research (CDER) United States Secondary...by Pill Pals Customer Service | Sep 16, 2025 | News
HOW TO USE THIS SNAPSHOT The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar...Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!

 VETMEDIN CHEW 10 MG --- Pimobendan --- Boehringer Ingelheim Pharma
VETMEDIN CHEW 10 MG --- Pimobendan --- Boehringer Ingelheim Pharma
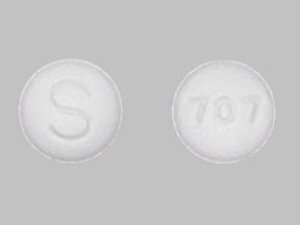
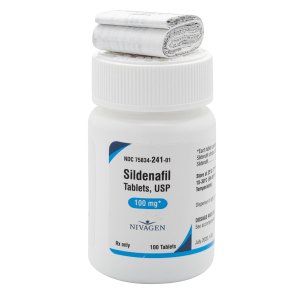 Sildenafil 100 mg Tablets --- Generic for Viagra --- Nivagen Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 100 mg Tablets --- Generic for Viagra --- Nivagen Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
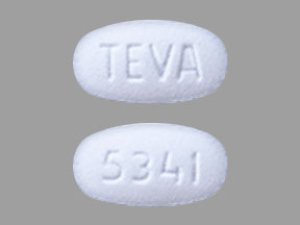 Sildenafil 25 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 25 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
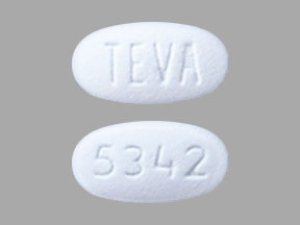 Sildenafil 50 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 50 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
 Docusate Sodium /Senna 8.6-50 mg Tablets 100 Count --- Generic for Peri-Colace --- Rugby Pharma
Docusate Sodium /Senna 8.6-50 mg Tablets 100 Count --- Generic for Peri-Colace --- Rugby Pharma