BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Oct 29, 2025 | News
SOPP 8401: Administrative Processing of Original Biologics License Applications (BLA) and New Drug Applications (NDA)Effective Date: January 15, 2025SOPP 8401.1: Issuance of and Review of Responses to Information Request Communications to Pending ApplicationsEffective...by Pill Pals Customer Service | Oct 29, 2025 | News
Docket Number: FDA-2011-D-0605 Issued by: Guidance Issuing Office Center for Drug Evaluation and Research, Office of New Drugs, Office of Therapeutic Biologics and Biosimilars FDA is posting this document to provide advance notice to the public. After the lapse in...by Pill Pals Customer Service | Oct 29, 2025 | News
On This Page Date: November 6, 2025 Time: 9:00 a.m. – 3:00 p.m. ET Location: Event Location FDA Headquarters FDA White Oak Campus10903 New Hampshire Ave, Building 31The Great RoomSilver Spring, MD 20903United States The 17th Annual Sentinel Initiative Public...by Pill Pals Customer Service | Oct 28, 2025 | News
Summary Company Announcement Date: October 27, 2025 FDA Publish Date: October 28, 2025 Product Type: Food & Beverages Contaminants Reason for Announcement: Recall Reason Description Potential Metal Contaminant – Lead Company Name: Homeneeds Inc. Brand Name:...by Pill Pals Customer Service | Oct 28, 2025 | News
Summary Company Announcement Date: October 28, 2025 FDA Publish Date: October 28, 2025 Product Type: Food & Beverages Foodborne Illness Reason for Announcement: Recall Reason Description Potential Foodborne Illness – Salmonella contamination Company Name: Pacific...by Pill Pals Customer Service | Oct 28, 2025 | News
On This Page Date: November 6, 2025 Time: 9:00 a.m. – 3:00 p.m. ET Location: Event Location FDA Headquarters FDA White Oak Campus10903 New Hampshire Ave, Building 31The Great RoomSilver Spring, MD 20903United States The 17th Annual Sentinel Initiative Public...by Pill Pals Customer Service | Oct 28, 2025 | News
Summary Company Announcement Date: October 28, 2025 FDA Publish Date: October 28, 2025 Product Type: Dietary Supplements Reason for Announcement: Recall Reason Description Potential contamination with Escherichia coli O7:K1 and 1303 Company Name: Dwater Brand Name:...by Pill Pals Customer Service | Oct 28, 2025 | News
Summary Company Announcement Date: October 25, 2025 FDA Publish Date: October 28, 2025 Product Type: Food & Beverages Reason for Announcement: Recall Reason Description May contain undeclared milk. Company Name: Teasdale Foods, Inc. Brand Name: Brand Name(s)...by Pill Pals Customer Service | Oct 28, 2025 | News
The Division of Pediatrics and Maternal Health oversees quality initiatives which promote and necessitate the study of drug and biological products in the pediatric population and improve safety data collection and product labeling for pregnant and lactating...Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
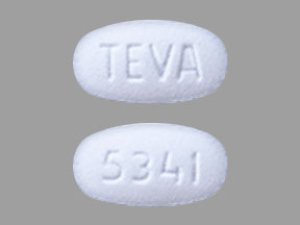 Sildenafil 25 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 25 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
 Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 5 --- Perrigo Pharma
Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 5 --- Perrigo Pharma
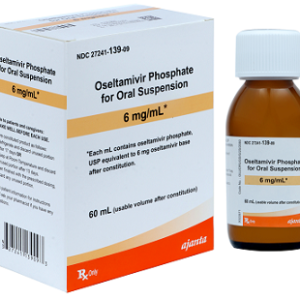 Oseltamivir Phosphate 6 mg / ml Oral Suspension --- Generic For Tamiflu (60 ml Total) --- Ajanta Pharma
Oseltamivir Phosphate 6 mg / ml Oral Suspension --- Generic For Tamiflu (60 ml Total) --- Ajanta Pharma
 Pataday - BRAND Eye Drops Allergy Itch Relief 0.2% by Alcon
Pataday - BRAND Eye Drops Allergy Itch Relief 0.2% by Alcon
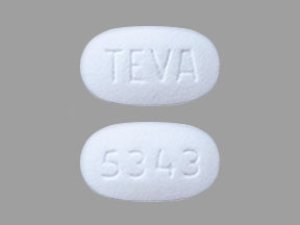 Sildenafil 100 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 100 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99