BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Jun 13, 2025 | News
This recall involves removing devices from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it.Affected Product MedicaLyte Liquid Bicarbonate Concentrate –...by Pill Pals Customer Service | Jun 12, 2025 | News
Summary Company Announcement Date: June 12, 2025 FDA Publish Date: June 12, 2025 Product Type: Food & Beverages Allergens Reason for Announcement: Recall Reason Description Potential or Undeclared Allergen – Sulfites Company Name: Turkana Food Inc. Brand...by Pill Pals Customer Service | Jun 12, 2025 | News
On June 12, 2025, the Food and Drug Administration approved pembrolizumab (Keytruda, Merck) for adults with resectable locally advanced head and neck squamous cell carcinoma (HNSCC) whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an...by Pill Pals Customer Service | Jun 12, 2025 | News
This page provides information about the electronic submission of regulatory information to the Center and the review of it by CDER staff. The Electronic Common Technical Document (eCTD) is the standard, accepted electronic format for the following...by Pill Pals Customer Service | Jun 12, 2025 | News
The following are a list of biological CBER compliance programs:Biologics7341.002: Inspection of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps)7342.001: Inspection of Licensed and Unlicensed Blood Banks, Brokers, Reference Laboratories, and...by Pill Pals Customer Service | Jun 12, 2025 | News
FDA would like to assist sponsors and applicants who have not previously submitted in eCTD v4.0. We offer a process to validate sample new eCTD v4.0 submissions and standardized study datasets. You must have an NDA, IND, BLA, ANDA, or MF number and plan to submit an...by Pill Pals Customer Service | Jun 12, 2025 | News
This list reflects information regarding the applications as of the approval date. It is not updated with regard to applicant or application status changes. The applications are listed by date of approval.2025 Biological License Application ApprovalsTradename/Proper...by Pill Pals Customer Service | Jun 12, 2025 | News
CDER would like to assist sponsors and applicants who have not previously submitted standardized study data and who are planning a submission to CDER. We offer a process to validate sample standardized study datasets. You must have an NDA, IND, BLA, ANDA, or DMF...by Pill Pals Customer Service | Jun 12, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: National Animal Disease Center, US Department of Agriculture Agricultural...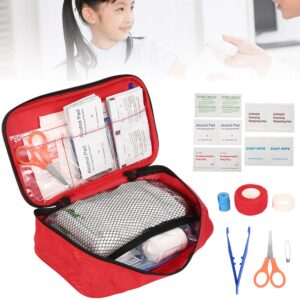 Portable Outdoor First Aid Kit
$7.99 – $29.99Price range: $7.99 through $29.99
Portable Outdoor First Aid Kit
$7.99 – $29.99Price range: $7.99 through $29.99
 Deep Bass Over Ear Headset
$137.99 – $141.99Price range: $137.99 through $141.99
Deep Bass Over Ear Headset
$137.99 – $141.99Price range: $137.99 through $141.99
 Men's Sport Chronograph Smartwatch
Men's Sport Chronograph Smartwatch
 Natural Blue Smelting Stone
$9.99 – $10.99Price range: $9.99 through $10.99
Natural Blue Smelting Stone
$9.99 – $10.99Price range: $9.99 through $10.99
 Dog Harness and Leash Set With Nylon Handle
$17.99 – $28.99Price range: $17.99 through $28.99
Dog Harness and Leash Set With Nylon Handle
$17.99 – $28.99Price range: $17.99 through $28.99