BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | May 28, 2025 | News
Submit Comments by 07/28/2025 Although you can comment on any guidance at any time (see 21 CFR 10.115(g)(5)), to ensure that the FDA considers your comment on a draft guidance before it begins work on the final version of the guidance, submit either online or written...by Pill Pals Customer Service | May 27, 2025 | News
On This Page Date: July 11, 2025 Time: 9:00 a.m. – 2:00 p.m. ET The Food and Drug Administration (FDA or Agency) is announcing a public meeting to discuss proposed recommendations for the reauthorization of the Generic Drug User Fee Amendments (GDUFA) for fiscal...by Pill Pals Customer Service | May 27, 2025 | News
1Columbia University Medical CenterNew York, New York2Brigham and Women’s HospitalBoston, Massachusetts8University of Kansas Medical CenterKansas City, Kansas10University of PennsylvaniaPhiladelphia, Pennsylvania11University of Virginia Health...by Pill Pals Customer Service | May 27, 2025 | News
Summary Company Announcement Date: May 22, 2025 FDA Publish Date: May 27, 2025 Product Type: Food & Beverages Foodborne Illness Reason for Announcement: Recall Reason Description Potential for Salmonella contamination. Company Name: The Coastal Companies Brand...by Pill Pals Customer Service | May 27, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Culmen International, LLC, Alexandria, Virginia, USA (G.C. Kelly, L.K....by Pill Pals Customer Service | May 27, 2025 | News
Date Issued: April 24, 2025The U.S. Food and Drug Administration (FDA) is alerting health care providers and laboratory staff of reports that falsely elevated (false positive) results have occurred when using ASP Global’s RAM Scientific SAFE-T-FILL Micro Capillary...by Pill Pals Customer Service | May 27, 2025 | News
Delivery Method: Via Email Product: Drugs Recipient: Shenzhen Hengkaifeng Commerce and Trade Co., Ltd 3A-18, Wansheng Baihuo, Wanzhong City, Xinniu Community, Minzhi Sub-DistrictLonghua QuShenzhen ShiGuangdong Sheng, 518000China zzkk15944@gmail.com Issuing Office:...by Pill Pals Customer Service | May 27, 2025 | News
Delivery Method: VIA UPS Reference #: 320-25-72 Product: Drugs Recipient: Recipient Name Mr. Atul B. Mehta Recipient Title President, Co-Chairman, and Co-Founder AACE Pharmaceuticals, Inc. 15 Gardner RoadFairfield, NJ 07004United States Issuing Office: Center for Drug...by Pill Pals Customer Service | May 27, 2025 | News
AUDIENCE: Consumer, Patient, Health Care Professional, Pharmacy, DermatologyISSUE: The FDA is warning that patients stopping the oral allergy medicines cetirizine (Zyrtec) or levocetirizine (Xyzal) after long-term use may experience rare but severe itching. These... Sildenafil 100 mg Tablets --- Generic for Viagra --- Ajanta Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
Sildenafil 100 mg Tablets --- Generic for Viagra --- Ajanta Pharma
$29.99 – $89.99Price range: $29.99 through $89.99
 Crystal Candle Holders for Home Decoration
$19.99 – $40.99Price range: $19.99 through $40.99
Crystal Candle Holders for Home Decoration
$19.99 – $40.99Price range: $19.99 through $40.99
 Rechargeable Night Fishing LED Headlamp
$10.99 – $21.99Price range: $10.99 through $21.99
Rechargeable Night Fishing LED Headlamp
$10.99 – $21.99Price range: $10.99 through $21.99
 Brushed Nickel Stylish Accessories for Bathroom
$8.99 – $60.99Price range: $8.99 through $60.99
Brushed Nickel Stylish Accessories for Bathroom
$8.99 – $60.99Price range: $8.99 through $60.99
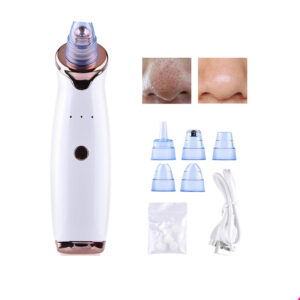 Electric Blackhead Remover Nose Cleaner
$11.99 – $17.99Price range: $11.99 through $17.99
Electric Blackhead Remover Nose Cleaner
$11.99 – $17.99Price range: $11.99 through $17.99