BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Feb 11, 2026 | News
Delivery Method: VIA UPS Reference #: 320-26-38 Product: Drugs Over-the-Counter Drugs Recipient: Recipient Name Ms. Maria Esparza Recipient Title President and Owner Signature Formulations, LLC 5446 W Roosevelt St., Ste 101Phoenix, AZ 85043United States Issuing...by Pill Pals Customer Service | Feb 11, 2026 | News
Delivery Method: VIA UNITED PARCEL SERVICE AND VIA E-MAIL Reference #: 26-HFD-45-02-01 Product: Drugs Recipient: Mark S. Dacey, M.D. 425 South Cherry Street, Suite 907Denver, CO 80246-1242United States Issuing Office: Center for Drug Evaluation and Research (CDER)...by Pill Pals Customer Service | Feb 11, 2026 | News
Delivery Method: Via Email Product: Drugs Issuing Office: Center for Drug Evaluation and Research (CDER) United States WARNING LETTERFebruary 5, 2026RE: 719715Dear James Nabors III:This letter is to advise you that the U.S. Food and Drug Administration (FDA) reviewed...by Pill Pals Customer Service | Feb 11, 2026 | News
Delivery Method: VIA UPS Reference #: 320-26-42 Product: Drugs Over-the-Counter Drugs Recipient: Recipient Name Mr. Yanzhuan Zheng Recipient Title Owner/President Bio-Medical Pharmaceutical Manufacturing Corporation 4311 South DriveHouston, TX 77053United States...by Pill Pals Customer Service | Feb 11, 2026 | News
Delivery Method: VIA Electronic Mail Product: Drugs Recipient: Recipient Name John Howell Recipient Title Executive Vice President Boothwyn Pharmacy, LLC 221 Gale LaneKennett Square, PA 19348United States Issuing Office: Center for Drug Evaluation and Research (CDER)...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
 Azithromycin 500 mg Tablet Three (3) Pack TAGI Pharma --- Generic For Z Pack / Zithromax
Azithromycin 500 mg Tablet Three (3) Pack TAGI Pharma --- Generic For Z Pack / Zithromax
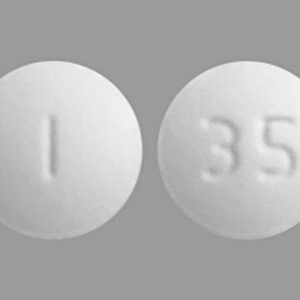 Sildenafil 25 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 25 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
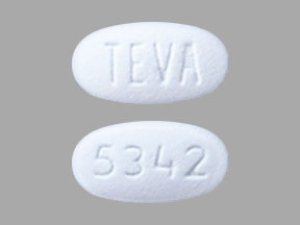 Sildenafil 50 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 50 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
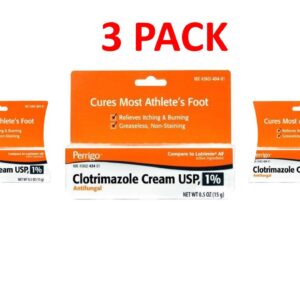 Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 3 --- Perrigo Pharma
Clotrimazole 1 Percent (1%) Cream 15g (0.5 oz) --- Generic for Lotrimin AF Pack of 3 --- Perrigo Pharma
 Acetaminophen 500 MG Caplets ---1,000 Count --- Generic For Tylenol --- Major Pharma
Acetaminophen 500 MG Caplets ---1,000 Count --- Generic For Tylenol --- Major Pharma