BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS ... Ready to order? Have Your Doctor Fax Your Prescription to 855.746.PALS. Dismiss
by Pill Pals Customer Service | Feb 13, 2026 | News
[2-13-2026] The Food and Drug Administration is advising consumers not to purchase or use Fantasy Aphrodisiac Chocolate, a product promoted and sold for sexual enhancement on various websites, including gearisle.com, and possibly in some retail stores. FDA laboratory...by Pill Pals Customer Service | Feb 13, 2026 | News
[2-13-2026] The Food and Drug Administration is advising consumers not to purchase or use LOVION Chocolate with Ginseng for Men, a product promoted and sold for sexual enhancement on various websites, including lovionusa.com, and possibly in some retail stores. FDA...by Pill Pals Customer Service | Feb 13, 2026 | News
This recall involves updating instructions for using devices, and does not involve removing them from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it...by Pill Pals Customer Service | Feb 13, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Author affiliations: National Centre for Disease Control and Prevention,...by Pill Pals Customer Service | Feb 13, 2026 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Author affiliations: San Diego Zoo Wildlife Alliance, San Diego,...by Pill Pals Customer Service | Feb 13, 2026 | News
The prescribing information has been updated on these products. Content current as of: 02/12/2026...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
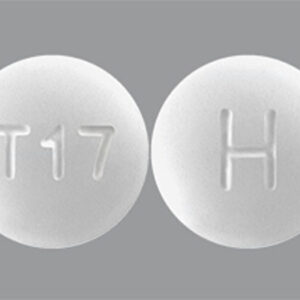 Tadalafil 5 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Tadalafil 5 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
 Topiramate 100 mg Tablet --- Generic For Topamax --- Zydus Pharma
$9.99 – $29.99Price range: $9.99 through $29.99 - or Auto-Reorder and Save 5%
Topiramate 100 mg Tablet --- Generic For Topamax --- Zydus Pharma
$9.99 – $29.99Price range: $9.99 through $29.99 - or Auto-Reorder and Save 5%
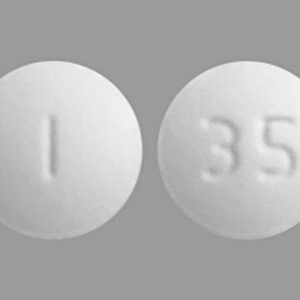 Sildenafil 25 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 25 mg Tablets --- Generic for Viagra --- Camber Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
 Tadalafil 10 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
Tadalafil 10 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
 Albuterol 90 MCG Inhaler --- Equivalent to Ventolin --- Prasco Pharma
Albuterol 90 MCG Inhaler --- Equivalent to Ventolin --- Prasco Pharma