by Pill Pals Customer Service | Dec 5, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Brigham and Women’s Hospital, Boston, Massachusetts, USA (K. Seung, C....by Pill Pals Customer Service | Dec 5, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Pasteur Institute, São Paulo, Brazil (E.S. Bergo, J. Telles-de-Deus, L.F....by Pill Pals Customer Service | Dec 4, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Author affiliation: University of North Carolina at Chapel Hill, Chapel...by Pill Pals Customer Service | Dec 4, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Pennsylvania Department of Health, Harrisburg, Pennsylvania, USA (N.M....by Pill Pals Customer Service | Dec 4, 2025 | News
HOW TO USE THIS SNAPSHOTThe information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar...by Pill Pals Customer Service | Dec 4, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Virginia Polytechnic Institute and State University, Blacksburg, Virginia,...by Pill Pals Customer Service | Dec 4, 2025 | News
On This Page Date: February 23, 2026 Time: 9:00 a.m. – 4:00 p.m. ET Image SummaryFDA will host Rare Disease Day, a virtual public meeting, on Monday, February 23, 2026 in global observance of Rare Disease Week. The theme is: “Moving Forward. Looking Ahead. An...by Pill Pals Customer Service | Dec 4, 2025 | News
On December 4, 2025, the Food and Drug Administration approved lisocabtagene maraleucel (Breyanzi, Juno Therapeutics, Inc., a Bristol-Myers Squibb Company) for adults with relapsed or refractory marginal zone lymphoma (MZL) who have received at least two prior lines...by Pill Pals Customer Service | Dec 4, 2025 | News
Identifying the Bacteria in Clinical Samples Patient blood samples were sent to the Pennsylvania Department of Health (PADOH)’s Bureau of Laboratories for bacterial identification and to rule out Brucella species, according to standard Laboratory Response Network...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
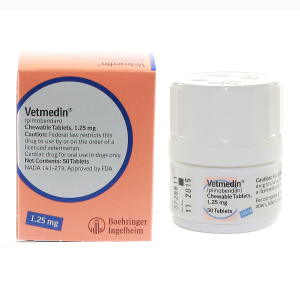 VETMEDIN CHEW 1.25MG --- Pimobendan --- Boehringer Ingelheim Pharma
VETMEDIN CHEW 1.25MG --- Pimobendan --- Boehringer Ingelheim Pharma
 Ibuprofen Liquid - Generic for Motrin 1 to 8 ounces
Ibuprofen Liquid - Generic for Motrin 1 to 8 ounces
 Sildenafil 100 mg Tablets --- Generic for Viagra --- Ajanta Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 100 mg Tablets --- Generic for Viagra --- Ajanta Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
 VETMEDIN CHEW 10 MG --- Pimobendan --- Boehringer Ingelheim Pharma
VETMEDIN CHEW 10 MG --- Pimobendan --- Boehringer Ingelheim Pharma
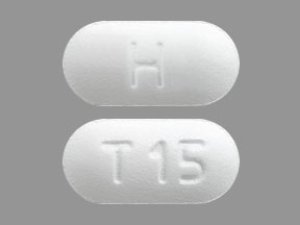 Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%
Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00 - or Auto-Reorder and Save 5%