by Pill Pals Customer Service | Dec 2, 2025 | News
FDA warns consumers that using unapproved ayurvedic products containing harmful levels of heavy metals may cause heavy metal poisoning. Using these products may result in high blood pressure, kidney injury, fatigue, gastrointestinal distress and neurologic symptoms....by Pill Pals Customer Service | Dec 2, 2025 | News
Summary Company Announcement Date: November 26, 2025 FDA Publish Date: December 02, 2025 Product Type: Animal & Veterinary Reason for Announcement: Recall Reason Description Presence of particulate matter Company Name: Vetoquinol USA Brand Name: Product...by Pill Pals Customer Service | Dec 2, 2025 | News
Docket Number: FDA-2025-D-4634 Issued by: Guidance Issuing Office Center for Drug Evaluation and Research The Food and Drug Administration (FDA or Agency) is announcing the availability of a draft guidance for industry entitled “Monoclonal Antibodies: Streamlined...by Pill Pals Customer Service | Dec 2, 2025 | News
Delivery Method: Via Electronic Mail – Return Receipt Requested Reference #: 320-26-17 Product: Drugs Recipient: Recipient Name Mr. Glen S. Putnam Recipient Title Senior Director of Global Quality Rhyz Analytical Labs 75 West Center St.Provo, UT 84601-4432United...by Pill Pals Customer Service | Dec 2, 2025 | News
Delivery Method: Via Electronic Mail – Delivery and Read Receipt Requested Product: Drugs Recipient: Recipient Name Laura Martin Turbare Manufacturing 925 Jeanette DriveConway, AR 72032-6651United States Issuing Office: Center for Drug Evaluation and Research...by Pill Pals Customer Service | Dec 2, 2025 | News
On This Page Date: December 11, 2025 Time: 12:00 p.m. – 1:00 p.m. ET Speaker Marie Bradley, PhDSenior Advisor for Real-World Evidence Office of Medical PolicyCenter for Drug Evaluation and Research U.S. Food and Drug AdministrationAbout the SpeakerDr. Marie...by Pill Pals Customer Service | Dec 2, 2025 | News
Delivery Method: Via Electronic Mail – Delivery and Read Receipt Requested Product: Drugs Recipient: Recipient Name Hale N. Dimetry Recipient Title President PQ Pharmacy, LLC 15215 Technology DriveBrooksville, FL 34604-0690United States Issuing Office: Center...by Pill Pals Customer Service | Dec 2, 2025 | News
This communication is an FDA Early Alert. The FDA has become aware of a potentially high-risk issue. The FDA will keep the public informed and update this web page as significant new information becomes available.Affected ProductThe FDA is aware that Abbott Diabetes...by Pill Pals Customer Service | Dec 2, 2025 | News
Docket Number: FDA-2023-D-2439 Issued by: Guidance Issuing Office Oncology Center of Excellence Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance is intended to assist applicants with incorporating heart rate-corrected...
Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!



 VETMEDIN CHEW 10 MG --- Pimobendan --- Boehringer Ingelheim Pharma
VETMEDIN CHEW 10 MG --- Pimobendan --- Boehringer Ingelheim Pharma
 Acetaminophen Liquid 160 MG / 5 ML - Generic for Tylenol 1 to 8 ounces
Acetaminophen Liquid 160 MG / 5 ML - Generic for Tylenol 1 to 8 ounces
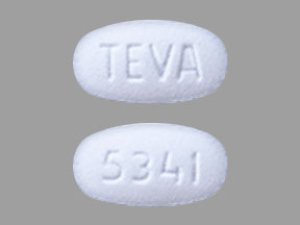 Sildenafil 25 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 25 mg Tablets --- Generic for Viagra --- TEVA Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
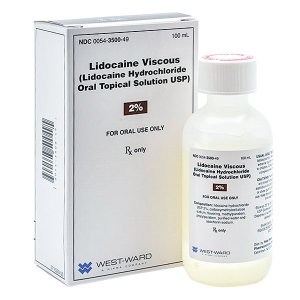 Lidocaine Viscous Solution Two Percent (2%) 100 ml --- Hikma Pharma
Lidocaine Viscous Solution Two Percent (2%) 100 ml --- Hikma Pharma
 Sildenafil 25 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%
Sildenafil 25 mg Tablets --- Generic for Viagra --- Torrent Pharma
$29.99 – $89.99Price range: $29.99 through $89.99 - or Auto-Reorder and Save 5%