BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Sep 16, 2025 | News
Delivery Method: Via Email Product: DrugsFood & Beverages Issuing Office: Human Foods Program United States July 17, 2025WARNING LETTERCMS #692733Dear Mr. Connelly:The U.S. Food and Drug Administration (FDA) conducted an inspection of your facility, located at...by Pill Pals Customer Service | Sep 16, 2025 | News
This recall involves correcting certain devices and does not involve removing them from where they are used or sold. The FDA has identified this recall as the most serious type. This device may cause serious injury or death if you continue to use it without...by Pill Pals Customer Service | Sep 16, 2025 | News
Delivery Method: Via UPS and EMAIL Reference #: CBER 25-707745 Product: Biologics Recipient: Recipient Name Park Thomas Recipient Title Chief Executive Officer and Owner NuVida Medical LLC 5814 S 25th StPhoenix, AZ 85040United States park.thomas@nuvidamed.com Issuing...by Pill Pals Customer Service | Sep 16, 2025 | News
Recipient: Recipient Name Kim Aubrey-Larcinese Recipient Title Senior Manager, Advertising & Promotion Global Regulatory Affairs CSL Behring 1020 First AvenueKing of Prussia, PA 19406United States Issuing Office: Center for Biologics Evaluation and Research...by Pill Pals Customer Service | Sep 15, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: US Centers for Disease Control and Prevention, Central Asia Office,...by Pill Pals Customer Service | Sep 15, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Centers for Disease Control and Prevention, Atlanta, Georgia, USA (X. Sun,...by Pill Pals Customer Service | Sep 15, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Author affiliations: Stanford University, Stanford, California, USA (A.B....by Pill Pals Customer Service | Sep 15, 2025 | News
CatalogCatalog# DescriptionUDI-DI1000911t:slim X2 GS, Replacement, Refurbished008530520073181003808t:slim X2 GS Classic, Replacement, Refurbished008530520079811004219t:slim X2, Basal-IQ, mg/dl008530520079981004484Pump, t:slim X2, Clinical Use OnlyN/A1005611Pump,...by Pill Pals Customer Service | Sep 15, 2025 | News
Summary Company Announcement Date: September 12, 2025 FDA Publish Date: September 15, 2025 Product Type: Medical Devices Reason for Announcement: Recall Reason Description Pump performance variations compared to the performance described in the user manual could...Click To Verify Security & Validation

Transferred Your Prescription?
 If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
If you have not already done so, please CLICK HERE to have your prescription transferred into the Pill Pals Pharmacy Network.
With Pill Pals, You Get The BEST CASH PRICES On ALL Meds!
 Azithromycin 500 mg Tablet Three (3) Pack TAGI Pharma --- Generic For Z Pack / Zithromax
Azithromycin 500 mg Tablet Three (3) Pack TAGI Pharma --- Generic For Z Pack / Zithromax
 Pataday - BRAND Eye Drops Allergy Itch Relief 0.2% by Alcon
Pataday - BRAND Eye Drops Allergy Itch Relief 0.2% by Alcon
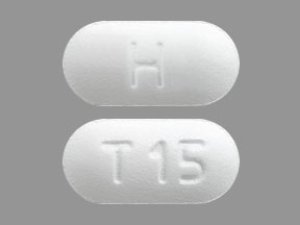 Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00
Tadalafil 20 MG Tablets --- Generic for Cialis --- Camber Pharma
$29.00 – $87.00Price range: $29.00 through $87.00
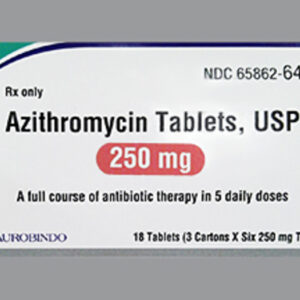 Azithromycin 250 mg Tablet Six (6) Pack --- Aurobindo Pharma --- Generic For Z Pack / Zithromax
Azithromycin 250 mg Tablet Six (6) Pack --- Aurobindo Pharma --- Generic For Z Pack / Zithromax
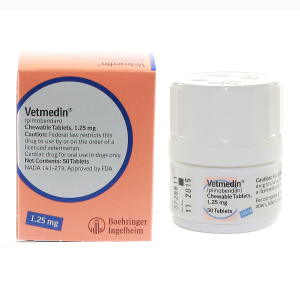 VETMEDIN CHEW 1.25MG --- Pimobendan --- Boehringer Ingelheim Pharma
VETMEDIN CHEW 1.25MG --- Pimobendan --- Boehringer Ingelheim Pharma