BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | Jun 11, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Radboud University Medical Center, Nijmegen, the Netherlands (F.Z. Delma,...by Pill Pals Customer Service | Jun 11, 2025 | News
The CDER Center for Clinical Trial Innovation (C3TI) Demonstration Program provides an opportunity to test and scale the integration of innovative approaches in clinical trials. The program offers a mechanism for early engagement between CDER and sponsors of clinical...by Pill Pals Customer Service | Jun 11, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Author affiliations: Interactions Hôtes-Agents Pathogènes, Université de...by Pill Pals Customer Service | Jun 11, 2025 | News
Strother D. Dixon, a senior regulatory project manager in OND’s Division of Dermatology and Dentistry, received a breast cancer diagnosis on Friday, March 13, 2009. Even with a biology background and pharmaceutical industry experience, she felt overwhelmed with...by Pill Pals Customer Service | Jun 11, 2025 | News
As part of the reauthorization of the Prescription Drug User Fee Act (PDUFA VII), FDA committed to reporting aggregate and anonymized information on submissions to the Center for Biologics Evaluation and Research (CBER) and the Center for Drug Evaluation and Research...by Pill Pals Customer Service | Jun 10, 2025 | News
Health care professionals can play a key role in educating patients about medication health fraud.Key Points to Know When Educating Patients about Medication Health FraudMedication health fraud refers to false or misleading claims about products’ ability to treat...by Pill Pals Customer Service | Jun 10, 2025 | News
Summary Company Announcement Date: June 10, 2025 FDA Publish Date: June 10, 2025 Product Type: Food & Beverages Foodborne Illness Reason for Announcement: Recall Reason Description Potential Foodborne Illness – Listeria monocytogenes Company Name: Bornstein...by Pill Pals Customer Service | Jun 10, 2025 | News
Medication health fraud refers to false or unproven claims about products’ ability to treat health issues, diseases or conditions. These products may also hide dangerous ingredients, including prescription drug ingredients, banned substances and/or harmful...by Pill Pals Customer Service | Jun 10, 2025 | News
FDA issues notifications to alert consumers when it finds products that are contaminated with hidden ingredients. These contaminated products are a type of medication health fraud and may cause you harm.You can find notifications about dangerous products organized by...
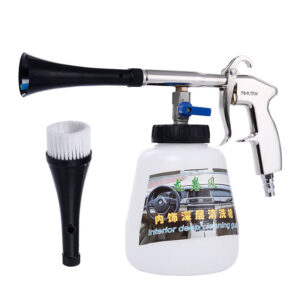 High Pressure Car Cleaning Sprayer
High Pressure Car Cleaning Sprayer
 Natural Black Short Kinky Curly Remy Human Hair Wig
Natural Black Short Kinky Curly Remy Human Hair Wig
 Ruffled Women's Lingerie Set in Black and White
$12.99 – $20.99Price range: $12.99 through $20.99
Ruffled Women's Lingerie Set in Black and White
$12.99 – $20.99Price range: $12.99 through $20.99
 Sexy Colorful Women's Lingerie Set with Deep V
$2.99 – $9.99Price range: $2.99 through $9.99
Sexy Colorful Women's Lingerie Set with Deep V
$2.99 – $9.99Price range: $2.99 through $9.99
 Lizard Shaped Statue for Garden Decor
Lizard Shaped Statue for Garden Decor