BEST CASH PRICES on Viagra, Levitra, Cialis + FREE SHIPPING ... JUST TEXT 855.816.PALS Dismiss
by Pill Pals Customer Service | May 21, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Chelsea G. Himsworth , Jessica M. Caleta, Agatha N. Jassem, Kevin C. Yang, James E.A. Zlosnik,...by Pill Pals Customer Service | May 21, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: The Connecticut Agricultural Experiment Station, New Haven, Connecticut,...by Pill Pals Customer Service | May 21, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Borrelia Lineages Adjacent to Zoonotic Clades in Black Flying Foxes (Pteropus alecto),...by Pill Pals Customer Service | May 21, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Hainan General Hospital, Affiliated Hainan Hospital of Hainan Medical...by Pill Pals Customer Service | May 21, 2025 | News
Each year, FDA consults with industry and the public to develop a list of regulatory science initiatives on generic drugs in accordance with the Generic Drug User Fee Amendments (GDUFA). These priorities are discussed and developed in public meetings and workshops and...by Pill Pals Customer Service | May 21, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. To the Editor: We congratulate Perciaccante et al. (1) on their article about a Paczka...by Pill Pals Customer Service | May 21, 2025 | News
Approved Active Moieties to which FDA has issued a Written Request for Pediatric Studies under Section 505A of the Federal Food, Drug, and Cosmetic ActNOTE: This list simply identifies approved active moieties to which FDA has issued a Written Request for pediatric...by Pill Pals Customer Service | May 21, 2025 | News
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released. Author affiliation: Abu Dhabi Agriculture and Food Safety Authority, Abu Dhabi, United Arab...by Pill Pals Customer Service | May 21, 2025 | News
For Immediate Release: May 21, 2025 The U.S. Food and Drug Administration is continuing to take steps to help state importation programs provide safe, effective and more affordable drugs for American patients, as part of its efforts to implement Executive Order... 1-2 Person Single Layer Water Resistant Tent
1-2 Person Single Layer Water Resistant Tent
 Straw Cushion in Round Shape
$11.99 – $16.99Price range: $11.99 through $16.99
Straw Cushion in Round Shape
$11.99 – $16.99Price range: $11.99 through $16.99
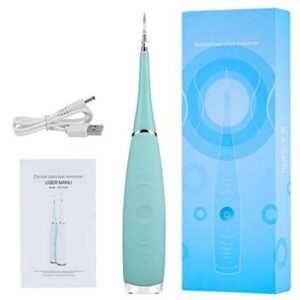 Portable Electric Dental Scaler Tool
$6.99 – $10.99Price range: $6.99 through $10.99
Portable Electric Dental Scaler Tool
$6.99 – $10.99Price range: $6.99 through $10.99
 Tube Design WiFi 1080P HD Dashcam with Voice Control
$41.99 – $69.99Price range: $41.99 through $69.99
Tube Design WiFi 1080P HD Dashcam with Voice Control
$41.99 – $69.99Price range: $41.99 through $69.99
 LCD Digital Measuring Spoon
$1.99 – $8.99Price range: $1.99 through $8.99
LCD Digital Measuring Spoon
$1.99 – $8.99Price range: $1.99 through $8.99